Let’s begin by stating the obvious. Caffeine occurs in coffee naturally, so any method of decaffeination (no matter how good it is) is considered by many aficionados unnatural. Some go even so far as claiming that it goes against the natural order of things…
Yet about 10% of coffee lovers would like to enjoy a good cup of coffee without the stimulating effect of caffeine. PS: I’ve written another article that covers other methods to cut down on caffeine.
Can it be done? Can you really have a GOOD cup of coffee that has been subjected to a decaffeination process? Read on and make up your own mind about whether it’s possible.
CAVEAT EMPTOR: “decaffeinated” does not mean 100% caffeine-free. In fact, a decaf coffee only needs to be 97% caffeine-free according to the USDA. That means that an average 12 oz. cup of decaffeinated coffee, which originally might have been packed with 180 mg of caffeine, now only would contain 5.4 mg of caffeine.
What All Decaffeination Processes Have in Common

Before we look at any specific decaffeination process let’s see what they share in common as a group.
- Coffee is always decaffeinated in its green (unroasted) state.
- The greatest challenge is to try to separate only the caffeine from the coffee beans while leaving the other chemicals at their original concentrations. This is not easy since coffee contains somewhere around 1,000 chemicals that are important to the taste and aroma of this wonderfully complex elixir.
- Since caffeine is a water-soluble substance, water is used in all forms of decaffeination.
- However, water by itself is not the best solution for decaffeination. Water is not a “selective” solvent and therefore removes other soluble substances, like sugars and proteins, as well as caffeine. Therefore, all decaffeination processes use a decaffeinating agent (such as methylene chloride, activated charcoal, CO2, or ethyl acetate). These agents help speed up the process and minimize the “washed-out” effects that water alone would have on the taste of decaf coffee.
The Roselius Process
 The first commercially successful decaffeination process was invented by the German coffee merchant Ludwig Roselius in 1903 and patented in 1906. Legend has it that his quest for decaffeinated coffee was motivated by the belief that excessive coffee drinking had poisoned his father.
The first commercially successful decaffeination process was invented by the German coffee merchant Ludwig Roselius in 1903 and patented in 1906. Legend has it that his quest for decaffeinated coffee was motivated by the belief that excessive coffee drinking had poisoned his father.
The “Roslius Process” involved steaming coffee beans with a brine solution (i.e., water, saturated with salt) and then using the organic chemical compound benzene as a solvent to extract the caffeine. This process, however, is no longer used due to the fact that benzene is known to be a human carcinogen.
The Four Main Methods of Decaffeination Used Today
In order to keep things nicely organized, let’s group the four processes into two general categories, each containing two methods.
Solvent-based processes |
Non-Solvent Based Processes |
|
|
Solvent based processes are those in which the caffeine is removed from the beans with the help of a chemical solvent, such as methylene chloride or ethyl acetate. Those solvent-based processes in turn can be divided into methods using the “direct” method versus the “indirect” method.
In the direct method, caffeine is removed by soaking the materials directly in a solvent; the solvent is directly applied to the beans. On the other hand, in the indirect method, the caffeine-laden water is transferred to a separate tank and treated with a solvent; in this case, the solvent never touches the beans.
Solvents used in decaffeination
As I’ve mentioned above, the decaffeination of coffee involves the use of a decaffeination agent. In solvent-based processes a chemical solvent is added either directly or indirectly to selectively remove the caffeine.
Given numerous health scares connected to early efforts in decaffeination (the hit list of toxic solvents includes: benzene, trichloroethylene (TCE), dichloromethane, and even chloroform) the solvents of choice have become methylene chloride and ethyl acetate.
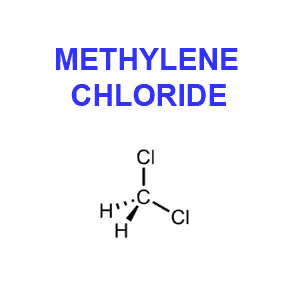 Although methylene chloride (CH2Cl2), aka dichloromethane (in Europe) or MC, is a solvent, its use as a decaffeination agent is not considered a health risk. In fact the Food and Drug Administration has determined any potential health risk is so low “as to be essentially non-existent” (FDA, 1985). While the FDA regulation allows up to ten parts per million (ppm) residual methylene chloride, actual coffee industry practice results in levels closer to one part per million.
Although methylene chloride (CH2Cl2), aka dichloromethane (in Europe) or MC, is a solvent, its use as a decaffeination agent is not considered a health risk. In fact the Food and Drug Administration has determined any potential health risk is so low “as to be essentially non-existent” (FDA, 1985). While the FDA regulation allows up to ten parts per million (ppm) residual methylene chloride, actual coffee industry practice results in levels closer to one part per million.
Furthermore, while it is probable that traces of the solvent remain in the decaffeinated beans it seems very unlikely that methylene chloride would survive the roasting process. This colorless liquid is highly volatile and vaporizes at 104 degrees F. Now if you take into consideration that coffee is roasted at a minimum of 400 degrees F for at least 15 minutes, and that proper brewing temperature is at about 200 degrees F, it seems unlikely that much if any methylene chloride would end up in your cup of Java.
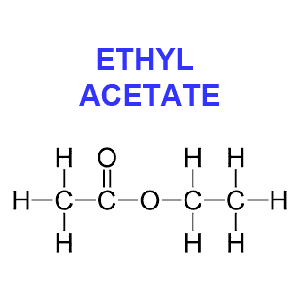 Ethyl acetate (CH3CO2C2H5) is hailed by some to be more “natural” than other chemicals and safer than methylene chloride since it exists in minute quantities in ripening fruits, such as apples and blackberries. Because this solvent occurs in nature you’ll often see coffee beans decaffeinated with this method labeled as “naturally” decaffeinated.
Ethyl acetate (CH3CO2C2H5) is hailed by some to be more “natural” than other chemicals and safer than methylene chloride since it exists in minute quantities in ripening fruits, such as apples and blackberries. Because this solvent occurs in nature you’ll often see coffee beans decaffeinated with this method labeled as “naturally” decaffeinated.
However, because of the impracticality of gathering natural ethyl acetate and its cost, the chemical used for decaffeination appears to be synthetic. Ethyl acetate is produced commercially from ethyl alcohol and acetic acid, which in turn may be produced from natural ingredients or petroleum derivatives.
Ethyl acetate, like methylene chloride, is very volatile and hence the point made above also applies here.
Now that we’ve got a basic framework to work with let’s have a closer look at the before mentioned methods. Since 70% of all coffee is decaffeinated with the help of solvents, I’ll start with solvent-based processes.
1) The Indirect–Solvent Based Process
In the indirect-solvent method, the coffee beans are soaked in near-boiling water for several hours, which extracts the caffeine as well as other flavor elements and oils from the beans.
The water is then separated and transferred to another tank where the beans are washed for about 10 hours with either methylene chloride or ethyl acetate. The molecules of the chemical solvent selectively bond with the molecules of caffeine and the resulting mixture is then heated to evaporate the solvent and caffeine.
Lastly, the beans are reintroduced to the liquid to reabsorb most of the coffee oils and flavor elements.
This method is very popular in Europe, especially in Germany, and primarily uses methylene chloride as a solvent. Hence it’s often referred to as “KVW Method” (short for “Kaffee Veredelugs Werk”), “The European Method,” “Methylene Chloride Method,” or “Euro Prep.”
2) The Direct–Solvent Based Process
In this method of decaffeination, the beans are steamed for about 30 minutes in order to open their pores. Once the coffee beans are receptive to a solvent, they are repeatedly rinsed with either methylene chloride or ethyl acetate for about 10 hours to remove the caffeine. The caffeine-laden solvent is then drained away and the beans are steamed again to remove any residual solvent.
Most of the time the solvent of choice in this method is ethyl acetate, so you’ll often see it referred to as “The Natural Decaffeination Method” or “The Ethyl Acetate Method.”
Typically, if a process is not named for a decaffeinated coffee, it has been treated by either the direct or indirect solvent methods.
1) The Swiss Water Process (SWP)
AKA: SWP Method, Swiss Water Process, Activated Charcoal Decaffeination, Dihydro-oxide Process
The History
This chemical-free water decaffeination process was pioneered in Switzerland in 1933 and developed as a commercially viable method of decaffeination by Coffex S.A. in 1980. In 1988 the Swiss Water Method was finally introduced to the market and its facility is based near Vancouver, British Columbia, Canada.
Note that the Swiss Water Company’s decaffeination facility is the only facility in the world certified organic by both OCIA and Aurora Certified Organic. In addition, they are also certified Kosher by the Kosher Overseers Association.
A Short Explanation of SWP
This particular method of decaffeination is different from what we’ve so far seen in that it does not directly or indirectly add chemicals to extract the caffeine. Rather, it relies entirely on two concepts, namely solubility, and osmosis, to decaffeinate coffee beans.
It begins by soaking a batch of beans in very hot water in order to dissolve the caffeine. The water is then drawn off and passed through an activated charcoal filter. The porosity of this filter is sized to only capture larger caffeine molecules while allowing smaller oil and flavor molecules to pass through it.
Consequently, we end up with beans with no caffeine and no flavor in one tank, and caffeine-free “flavor charged” water (aka “Green Coffee Extract”) in another tank.
And here’s where the magic happens. The flavorless caffeine-free beans are discarded, but the flavor-rich water is reused to remove the caffeine from a fresh batch of coffee beans.
Since this water already is saturated with flavor ingredients the flavors in this fresh batch can’t dissolve; only caffeine moves from the coffee beans to the water. So the result is decaffeination without a massive loss of flavor.
Coffees decaffeinated by this method are always labeled as “SWISS WATER” Decaf.
This method is almost exclusively used for the decaffeination of organic coffee.
Coffee decaffeinated using the environment-friendly Swiss Water Process undergoes regular caffeine level audits to ensure compliance to 99.9% caffeine-free.
2) CO2 process
AKA: CO2 (or Carbon Dioxide ) Method, Liquid Carbon Dioxide Method, Supercritical Carbon Dioxide Method
The Carbon Dioxide (CO2) Method is the most recent method. It was developed by Kurt Zosel, a scientist of the Max Plank Institute, and uses liquid CO2 in place of chemical solvents. It acts selectively on the caffeine, i.e., it releases the alkaloid and nothing else.
In the CO2 decaffeination process, water-soaked coffee beans are placed in a stainless steel container called the extraction vessel. The extractor is then sealed and liquid CO2 is forced into the coffee at pressures of 1,000 pounds per square inch to extract the caffeine.
The CO2 acts as the solvent to dissolve and draw the caffeine from the coffee beans, leaving the larger-molecule flavor components behind. The caffeine-laden CO2 is then transferred to another container called the absorption chamber. Here the pressure is released and the CO2 returns to its gaseous state, leaving the caffeine behind. The caffeine-free CO2 gas is pumped back into a pressurized container for reuse.
Because of its cost, this process is primarily used to decaffeinate large quantities of commercial-grade, less-exotic coffee found in grocery stores.
Why is it so Difficult to Make Good Decaf Coffee?
Let’s start by stating a sad fact first: finding a good decaf coffee is the exception rather than the norm. The reason for this centers around two problems that are very difficult to overcome.
First, as we’ve already seen, the decaffeination process tends to damage many flavor compounds that contribute to the sensory character of roasted coffee.

Secondly, decaf coffees are notoriously difficult to roast. The reason for this is that decaffeinated, unroasted coffee beans start off almost brown in color rather than green. This makes it difficult for the roasters to control them, since they respond inconsistently and exaggeratedly to heat applied to them during roasting. Furthermore, they have less bound moisture content, which causes them to roast faster. So, you dealing with an unroasted “green” bean that tends to roast darker and faster than un-decaffeinated beans.
But all is not lost. Given what we just learned we can use this information to ensure a better decaf experience. Overall the type of roast you buy is going to have more of an impact on the taste than the decaf method. Try to avoid any decaf coffees that are really dark and oily, for you certainly don’t want to add the ravages of a very dark roast to the rigors of any decaffeination process.
What’s your favorite decaf? Is there any particular process or roaster that you particularly like? Let’s hear it in the comments!
…and if you found this article valuable, go ahead and share it on your favorite social network 😉

